The debate about whether, when and how to use isotype controls appears to be controversial to researchers (Herzenberg L et al., Maecker HT and Trotter J.). You may not wish to use isotype controls, but if you do this article will give you an overview of what an isotype control is, when and how to use it.
Remember they are only one of a number of appropriate controls that will improve you flow cytometry data. For more information on controls in flow cytometry, we have a dedicated page, with a more detailed overview of controls in flow cytometry.
What are isotype controls?
Unwanted background cell staining in flow cytometry can be a problem, especially when detecting novel or rare populations and when building panels containing multiple fluorophores. An isotype control is an antibody raised against an antigen not present on the cell type being analyzed (e.g. KLH or DNP) and has been specifically developed to determine the level of background surface staining.
An isotype control will:
- Determine the non-specific binding of an antibody to Fc receptors found on monocytes, macrophages, dendritic and B cells
- Ensure the observed staining is due to specific binding rather than an artifact
- Reveal other non-specific binding of the antibody or fluorophores to cellular components (e.g. RPE and FITC, Takizawa et al, 1993, Hulspas et al. 2009)
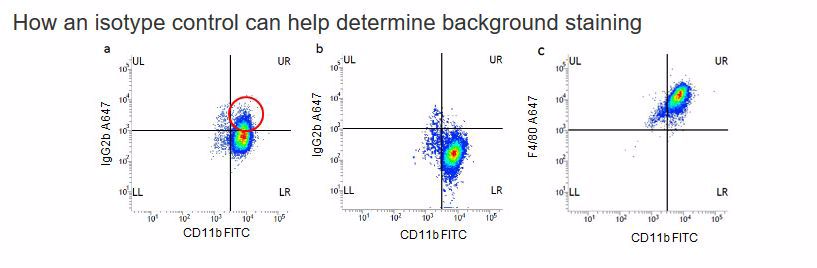
Fig 1. J774 macrophages were stained for 30 minutes at 4oC in PBS w/v1%BSA with CD11b FITC (MCA74F) and IgG2b Alexa Fluor®647 (MCA6006A647) a). In the absence of Fc block or b). In the presence of mouse Fc block (mouse Seroblock FCR, BUF041A). In Fig1a there is a significant population of cells positive (circled) with the A647 isotype control showing the Fc binding which disappears when Fc block is included, Fig1b. Fig1c shows specific F4/80 A647 (MCA497A647) staining. All data shown was gated on the 7-AAD negative, live population with the use of doublet discrimination.