The major histocompatibility complex (MHC) is comprised of cell membrane proteins that deliver short peptides to the cell surface, presenting these peptide antigens to circulating immune surveillance cells. These peptides are mostly self-peptides however during times of infection these can also be antigens from pathogens that, upon recognition and subsequent binding by the immune cells, specifically T cells, result in the generation of an immune response.
The major histocompatibility complex (MHC) is comprised of cell membrane proteins that deliver short peptides to the cell surface, presenting these peptide antigens to circulating immune surveillance cells. These peptides are mostly self-peptides however during times of infection these can also be antigens from pathogens that, upon recognition and subsequent binding by the immune cells, specifically T cells, result in the generation of an immune response.
The MHC-peptide complex on the cell membrane interacts with the T cell receptor (TCR) with the MHC binding to the TCR and its CD3/CD4 or CD8 co-receptors, while the antigenic epitope is bound by the variable Ig-like domain of the TCR. These interactions stimulate T cell activation. Read our mini-reviews for more detailed information on the TCR and CD3, the major T cell co-receptor.
The MHC gene expresses three types of molecule. The class III molecules are not involved in antigen presentation; instead they play a role in inflammation. These molecules include complement components such as C2 and C4, tumor necrosis factor - α and heat shock proteins. There are two main types of MHC that present antigens; MHC class I and MHC class II.
Due to the requirement of MHC interactions with TCR and its co-receptors in the appropriate activation of T cells, they also play a critical role in thymic T cell selection. The first stage in thymic T cell selection, positive selection, requires the T cells to bind to MHC Class I or MHC Class II molecules with an appropriate affinity to initiate the stimulation of “survival signals” within the T cell. The second stage of T cell selection, negative selection, removes auto-reactive T cells and, therefore prevents autoimmunity. During negative selection the majority of T cells that bind too strongly to self-antigens receive apoptotic signals which result in cell death. T cells with low affinity for self survive to become effector T cells, while those with an intermediate affinity for self may go on to become regulatory T cells. Only 2% of the thymocytes populating the thymus reach maturity and exit the thymus to circulate as immature T cells. In the periphery when self-antigen is presented to T cells, T cell activation should not take place. Autoimmune disease occurs when control of auto-recognition fails, and results in T cells being unable to tolerate self.
In humans the MHC is also known as human leukocyte antigen (HLA) complex and in the mouse it is known as the H-2 complex.
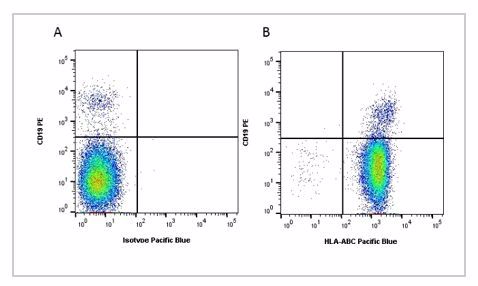
Fig.1. Flow cytometry analysis of Human HLA Marker HLA ABC. A, RPE conjugated Mouse Anti-Human CD19 (MCA1940PE) and Pacific Blue conjugated Mouse IgG2a Isotype Control (MCA929PB). B, RPE conjugated Mouse Anti-Human CD19 (MCA1940PE) and Pacific Blue conjugated Mouse Anti-Human HLA ABC (MCA81PB). All experiments performed on red cell lysed human blood gated on lymphocytes. Data acquired on the ZE5™ Cell Analyzer.
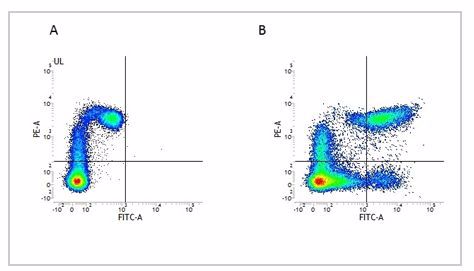
Fig.2. Flow cytometry analysis of Human HLA Marker HLA DP DQ DR. A, RPE conjugated Mouse Anti-Human CD11c (MCA2087PE) and FITC conjugated Mouse IgG2a Isotype Control (MCA929F). B, RPE conjugated Mouse Anti-Human CD11c (MCA2087PE) and FITC conjugated Mouse Anti-Human HLA DP/DQ/DR (MCA477F). All experiments performed on human peripheral blood mononuclear cells in the presence of Human SeroBlock (BUF070A).