FLISP Serine Protease Detection Kits

- On This Page
- Detecting apoptosis<
- Available FLISP kits
- References
Detecting Apoptosis
Apoptosis, a programmed cell death mechanism, is an essential process in embryogenesis and development. The process is also critical for ensuring an organism’s health by removing aberrant or damaged cells.
General levels of apoptosis, not specific to either the intrinsic or extrinsic pathways, can be measured in various ways. For example, annexin V and pSIVA can be used to detect exposure of phosphatidyl serine at the outer plasma membrane and Poly Caspase FLICA Kits for the detection of active caspases. Here we highlight another way to detect apoptosis in mammalian cells occuring downstream of caspase activation using FLISP Serine Protease Detection Kits (Grabarek et al. 2002).
Fluorescent Labeled Inhibitors of Serine Protease (FLISP) Kits allow the detection of intracellular chymotrypsin-like activity of serine proteases in live cells. Serine protease activation plays major roles in apoptosis (Johnson 2000), tumor malignancy (Chen et al. 2001, Magee et al. 2001) and as diagnostic indicators of breast (Yousef et al. 2000), head and neck carcinomas (Lang et al. 2001). They are also thought to play a role in infections and transplant rejection (Estanbanez-Perpina et al. 2000, Jans et al. 1998).
Active chymotrypsin-like enzymes within the cell bind covalently to the fluorophore (FAM) labeled FLISP inhibitor probe, retaining the green fluorescent signal within the cell. The higher the concentration of active chymotrypsin-like enzymes within the cell, the higher the level of fluorescence observed. An example of typical staining is shown in Figure 1. Jurkat cells were treated with staurosporine to induce apoptosis and after 6 hours significant levels of enzyme activity were detected. Apoptosis assays often require viability dyes to distinguish between necrotic and apoptotic cells in the experiment. FLISP Kits contain propidium iodide for dead cell identification by flow cytometry and Hoechst 33342 for nuclear labeling in fluorescence microscopy.
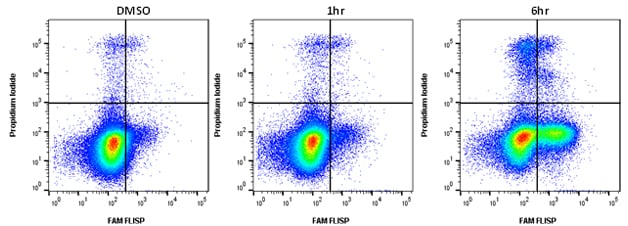
Fig.1. Active chymotrypsin-like serine protease activity staining by flow cytometry. Jurkat cells were treated as shown with 1 µM staurosporine to induce apoptosis. The cells were then stained using the FLISP Serine Protease Assay Kit including propidium iodide (APO009). Apoptotic cells positive for serine protease activity can be seen in the bottom right quadrant and dead cells positive for both serine protease and PI in the top right quadrant. Healthy cells are negative for both stains. Data acquired on the ZE5™ Cell Analyzer.
FLISP inhibitors are cell-permeable and non-cytotoxic fluorochrome-labeled analogs of the first serine protease inhibitor, tosyl phenylalanyl chloromethyl ketone (TPCK), labeled with carboxyfluorescein (FAM) which is excitable by a 488 nm laser and emits at 520 nm. They are available with either a chloromethyl ketone (CMK) or diphenyl 1-(N-peptidylamino) alkanephosphonate (DAP) reactive group containing compound. Specificity for chymotrypsin-like enzymes is determined by the amino acid targeting peptide. A Phe moiety will bind to chymotrypsin, whereas a Leu moiety will target chymotrypsin C.
Our FLISP Serine Protease Assay Kit Range
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
Any active chymotrypsin-like enzyme within a cell will covalently bind to FLISP, retaining it within the cell, and therefore apoptotic cells will fluoresce more brightly than non-apoptotic cells with fewer chymotrypsin-like enzymes. FLISP Kits may be used in combination with FLICA Kits to discriminate between caspase activity and serine protease activity. FLISP Kits are suitable for use with suspension or adherent cells and can be used with a flow cytometer, fluorescence microscope or a fluorescence plate reader.
Find more in-depth information on apoptosis by clicking on the links below
References
- Chen L-M et al. (2001). Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem 276, 21434-42.
- Estanbanez Perpina E et al. (2000). Crystal structure of the caspase activator human granzyme B, a proteinase highly specific for an Asp-P1 residue. Biol Chem 12, 1203-14.
- Grabarek et al. (2002). Sequential activation of caspases and serine proteases (serpases) during apoptosis. Cell Cycle 2, 124-131.
- Jans DA et al. (1998). Nuclear targeting of the serine protease granzyme A (fragmentin-1). J Cell Sci. Pt17, 2645-54.
- Johnson DE (2000). Noncaspase proteases in apoptosis. Leukemia 14, 1695-1703.
- Lang JC et al. (2001). Differential expression of a novel serine protease homologue in squamous cell carcinoma of the head and neck. Br J cancer 84, 237-43.
- Magee JA et al. (2001). Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res 15, 5692-6.
- Yousef GM et al. (2000). KLK12 is a novel serine protease and a new member of the human kallikrein gene family-differential expression in breast cancer. Genomics 3, 331-41.


